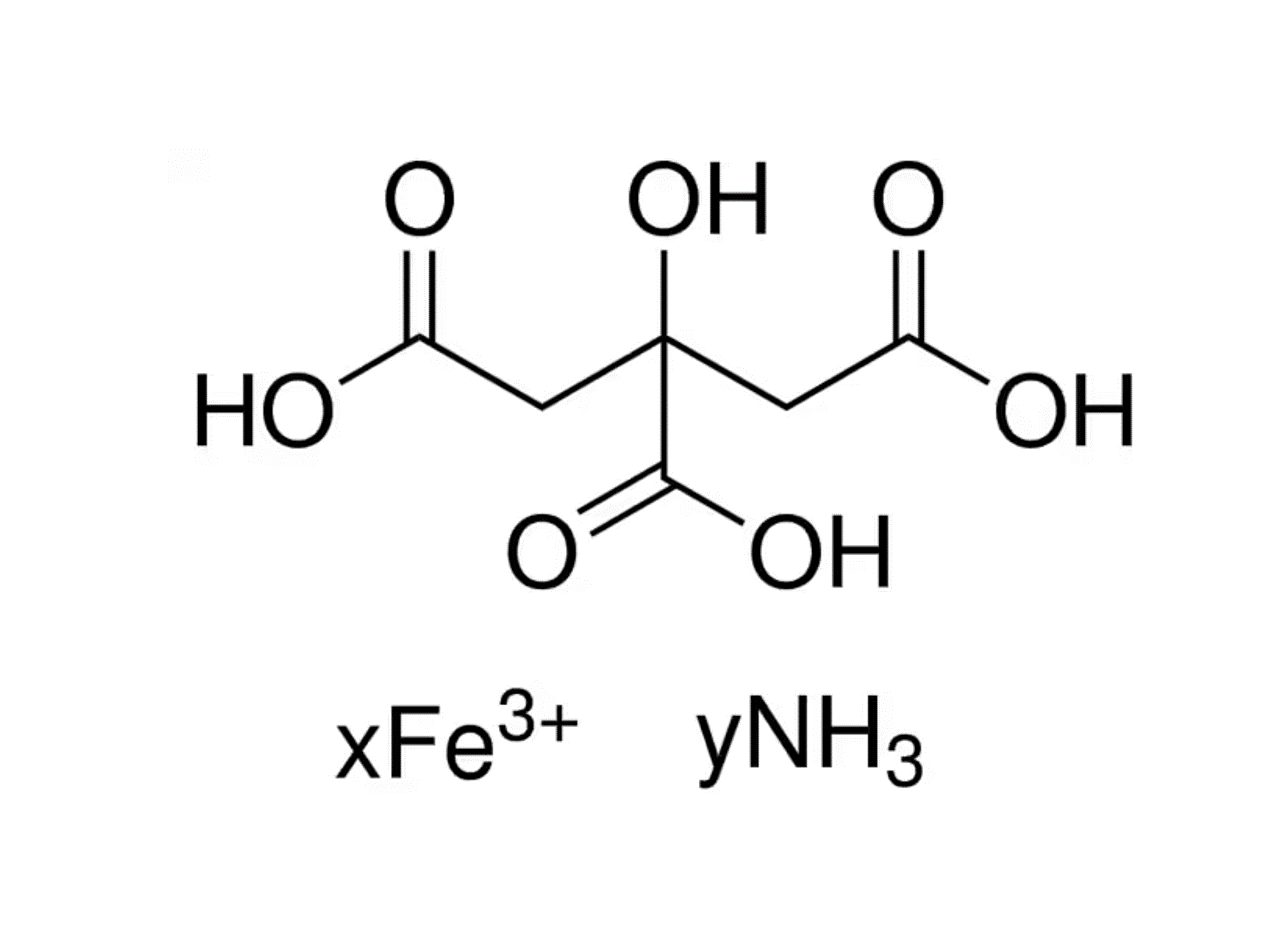
Ammonium ferric citrate (also known as ferric ammonium citrate or ammoniacal ferrous citrate) has the formula [NH4]y[Fex(C6H4O7)]. The iron in this compound is trivalent. All three carboxyl groups and the central hydroxyl group of citric acid are deprotonated. A distinguishing feature of this compound is that it is very soluble in water, in contrast to ferric citrate which is not very soluble.
In its crystal structure each moiety of citric acid has lost four protons. The deprotonated hydroxyl group and two of the carboxylate groups ligate to the ferric center, while the third carboxylate group coordinates with the ammonium.
Uses
Ammonium ferric citrate has a range of uses, including:
- As a food ingredient, it has an INS number 381, and is used as an acidity regulator. Most notably used in the Scottish beverage Irn-Bru.
- Water purification
- As a reducing agent of metal salts of low activity like gold and silver
- With potassium ferricyanide as part of the cyanotype photographic process
- Used in Kligler's Iron Agar (KIA) test to identify enterobacteriaceae bacteria by observing their metabolism of different sugars, producing hydrogen sulfide
- In medical imaging, ammonium ferric citrate is used as a contrast medium.
- As a hematinic
See also
- Food additive
- List of food additives
References